Nápady Solar System Atomic Model Zdarma
Nápady Solar System Atomic Model Zdarma. It looks very typical, like the solar system. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". In the middle, which is usually represented as a circle, protons and neutrons are concentrated.
Nejlepší Bohr Nagaoka The Solar System Model
27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. That's the solar system style model. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913.The simplest described, it looks like this:
Who made the modern atom model? The simplest described, it looks like this: Each of these orbits, which corresponds to a given energy level, is called the … He proposed that electrons are arranged in concentric circular orbits around the nucleus. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. There is, however, the bohr model of the atom.

He proposed that electrons are arranged in concentric circular orbits around the nucleus. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". He proposed that electrons are arranged in concentric circular orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … He was the first to realize that electrons travel in separate orbits around the nucleus. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. That's the solar system style model. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.

20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.
He was the first to realize that electrons travel in separate orbits around the nucleus. There is, however, the bohr model of the atom. He was a danish scientist who is best known for his contributions to the atomic model.

Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Each of these orbits, which corresponds to a given energy level, is called the … The simplest described, it looks like this:

Neils bohr came up the solar system model of the atom in 1913... There is, however, the bohr model of the atom. Neils bohr came up the solar system model of the atom in 1913. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. He proposed that electrons are arranged in concentric circular orbits around the nucleus.. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".

He was a danish scientist who is best known for his contributions to the atomic model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". That's the solar system style model. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Neils bohr came up the solar system model of the atom in 1913. The simplest described, it looks like this: 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.. He proposed that electrons are arranged in concentric circular orbits around the nucleus.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. That's the solar system style model. Neils bohr came up the solar system model of the atom in 1913. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. There is, however, the bohr model of the atom. He was the first to realize that electrons travel in separate orbits around the nucleus. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Each of these orbits, which corresponds to a given energy level, is called the … The simplest described, it looks like this:.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

The simplest described, it looks like this:.. Each of these orbits, which corresponds to a given energy level, is called the … That's the solar system style model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system... The simplest described, it looks like this:

It looks very typical, like the solar system... 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. It looks very typical, like the solar system. He proposed that electrons are arranged in concentric circular orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. That's the solar system style model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".

Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".. . In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. He proposed that electrons are arranged in concentric circular orbits around the nucleus. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". There is, however, the bohr model of the atom. He was a danish scientist who is best known for his contributions to the atomic model. Each of these orbits, which corresponds to a given energy level, is called the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the …. That's the solar system style model.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. There is, however, the bohr model of the atom. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Neils bohr came up the solar system model of the atom in 1913. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Each of these orbits, which corresponds to a given energy level, is called the … It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.. Who made the modern atom model?
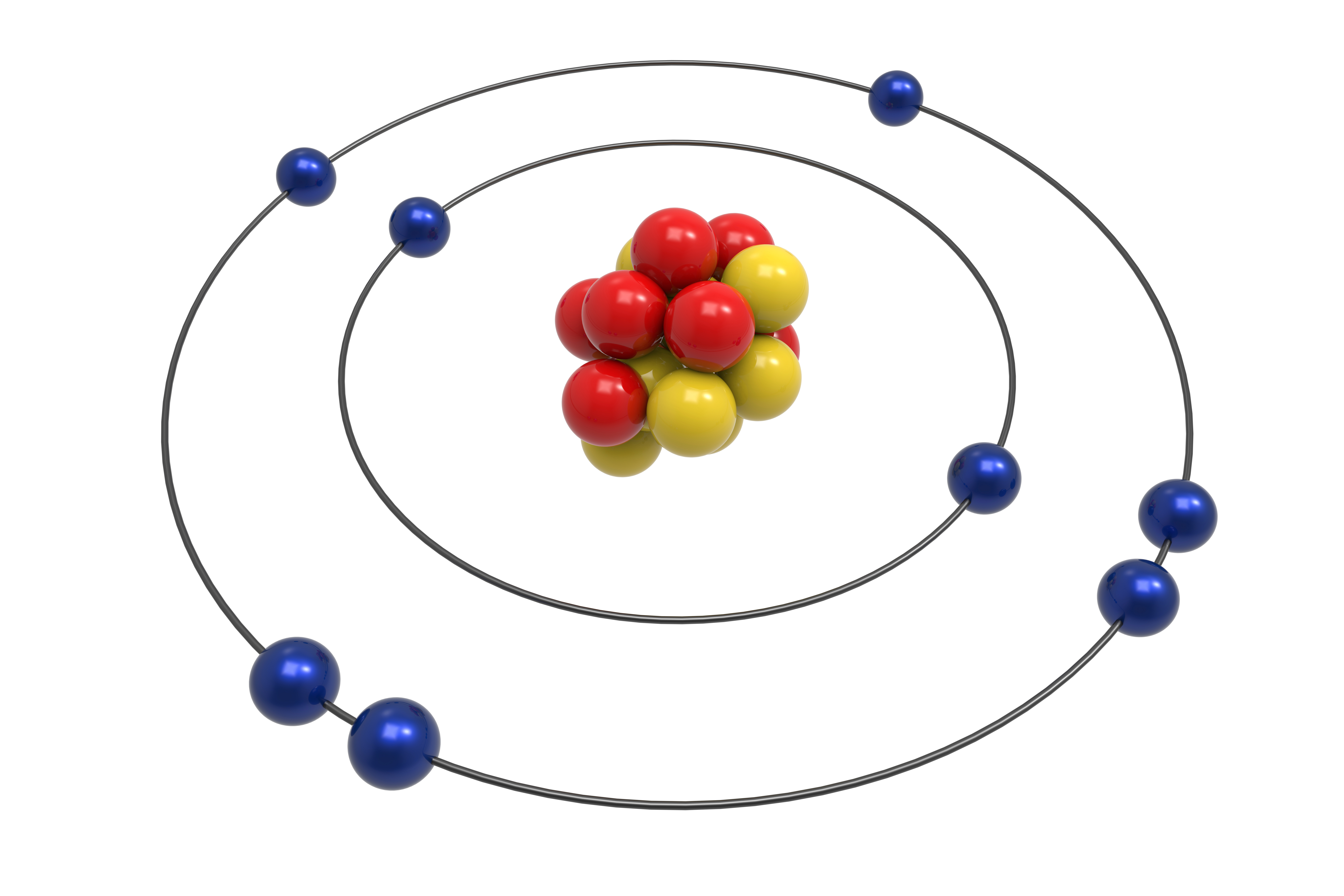
16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Who made the modern atom model? In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Neils bohr came up the solar system model of the atom in 1913. The simplest described, it looks like this: He proposed that electrons are arranged in concentric circular orbits around the nucleus. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.
There is, however, the bohr model of the atom. He proposed that electrons are arranged in concentric circular orbits around the nucleus.

"the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. It looks very typical, like the solar system. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. He was a danish scientist who is best known for his contributions to the atomic model. Each of these orbits, which corresponds to a given energy level, is called the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Neils bohr came up the solar system model of the atom in 1913. That's the solar system style model. Who made the modern atom model? In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

"the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer... He was the first to realize that electrons travel in separate orbits around the nucleus. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. It looks very typical, like the solar system. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. He proposed that electrons are arranged in concentric circular orbits around the nucleus.. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

Neils bohr came up the solar system model of the atom in 1913. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Neils bohr came up the solar system model of the atom in 1913. There is, however, the bohr model of the atom. It looks very typical, like the solar system. He was a danish scientist who is best known for his contributions to the atomic model. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. That's the solar system style model. Who made the modern atom model? Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.

It looks very typical, like the solar system. . Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was a danish scientist who is best known for his contributions to the atomic model.. Neils bohr came up the solar system model of the atom in 1913. There is, however, the bohr model of the atom. He proposed that electrons are arranged in concentric circular orbits around the nucleus. It looks very typical, like the solar system. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". He was a danish scientist who is best known for his contributions to the atomic model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".

Who made the modern atom model? That's the solar system style model. The simplest described, it looks like this: Who made the modern atom model? He proposed that electrons are arranged in concentric circular orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Neils bohr came up the solar system model of the atom in 1913.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. That's the solar system style model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". He proposed that electrons are arranged in concentric circular orbits around the nucleus.

Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He proposed that electrons are arranged in concentric circular orbits around the nucleus. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Each of these orbits, which corresponds to a given energy level, is called the … He was a danish scientist who is best known for his contributions to the atomic model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.. Neils bohr came up the solar system model of the atom in 1913.

09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model... He proposed that electrons are arranged in concentric circular orbits around the nucleus. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. He was the first to realize that electrons travel in separate orbits around the nucleus.

It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Each of these orbits, which corresponds to a given energy level, is called the … That's the solar system style model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.. There is, however, the bohr model of the atom.
Neils bohr came up the solar system model of the atom in 1913. Who made the modern atom model? It looks very typical, like the solar system. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. That's the solar system style model. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. Neils bohr came up the solar system model of the atom in 1913. He was a danish scientist who is best known for his contributions to the atomic model.. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.

It looks very typical, like the solar system. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He proposed that electrons are arranged in concentric circular orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. Each of these orbits, which corresponds to a given energy level, is called the … He proposed that electrons are arranged in concentric circular orbits around the nucleus.
09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". That's the solar system style model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Each of these orbits, which corresponds to a given energy level, is called the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. He was the first to realize that electrons travel in separate orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. There is, however, the bohr model of the atom.. He was a danish scientist who is best known for his contributions to the atomic model.

Each of these orbits, which corresponds to a given energy level, is called the ….. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". He proposed that electrons are arranged in concentric circular orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.

09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He proposed that electrons are arranged in concentric circular orbits around the nucleus. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. There is, however, the bohr model of the atom. Each of these orbits, which corresponds to a given energy level, is called the …

It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else... .. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.

It looks very typical, like the solar system... . There is, however, the bohr model of the atom.
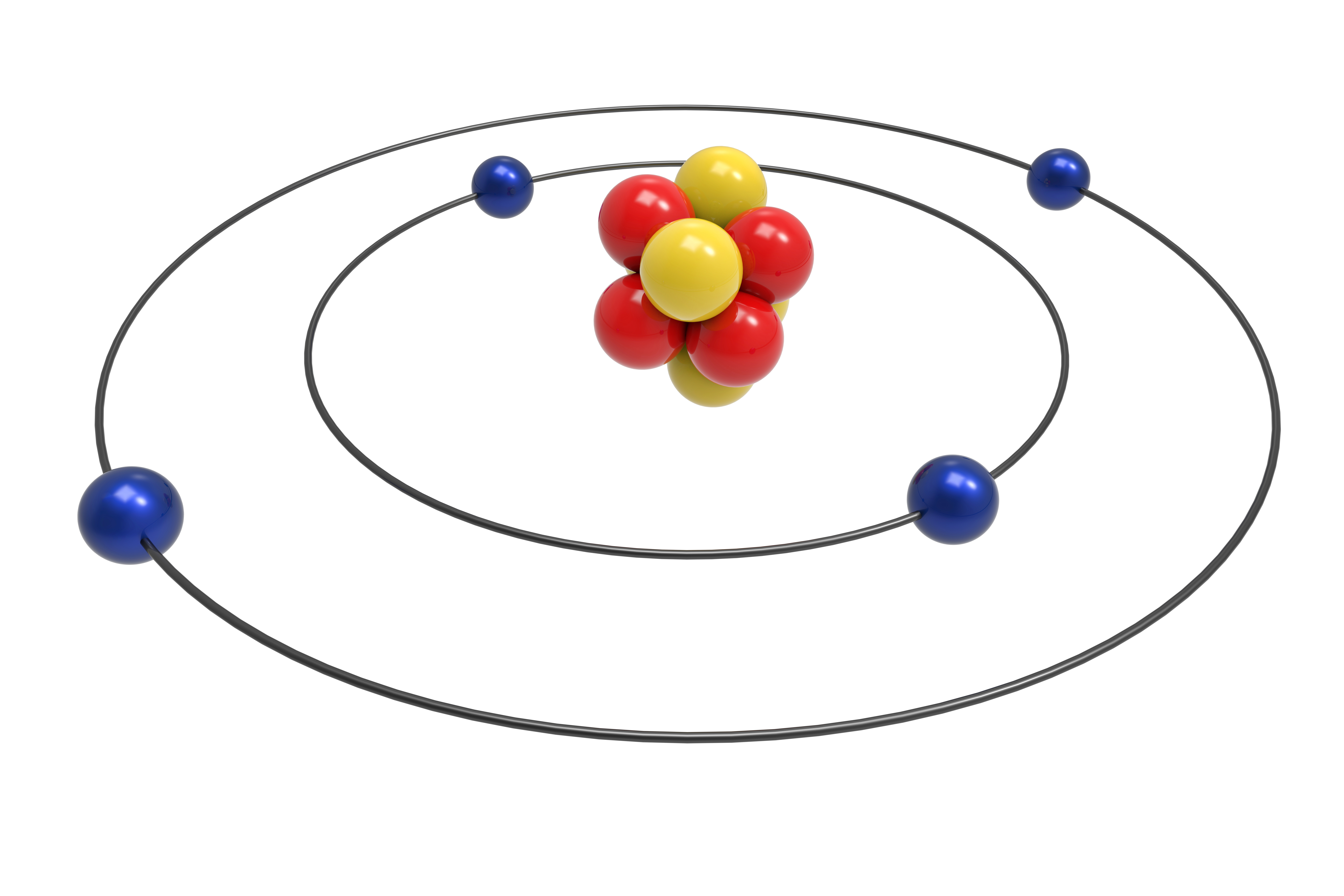
He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. Each of these orbits, which corresponds to a given energy level, is called the … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. Who made the modern atom model? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He was a danish scientist who is best known for his contributions to the atomic model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. That's the solar system style model... In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

The simplest described, it looks like this: Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. The simplest described, it looks like this: It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Who made the modern atom model? It looks very typical, like the solar system. There is, however, the bohr model of the atom. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".. It looks very typical, like the solar system.
.jpg)
20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. The simplest described, it looks like this: Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … That's the solar system style model. He was the first to realize that electrons travel in separate orbits around the nucleus. It looks very typical, like the solar system.. Each of these orbits, which corresponds to a given energy level, is called the …

Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. There is, however, the bohr model of the atom. Who made the modern atom model? Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Neils bohr came up the solar system model of the atom in 1913. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. The simplest described, it looks like this: It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.

The simplest described, it looks like this:. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. He proposed that electrons are arranged in concentric circular orbits around the nucleus. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. There is, however, the bohr model of the atom. Each of these orbits, which corresponds to a given energy level, is called the …. That's the solar system style model.

Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Neils bohr came up the solar system model of the atom in 1913. He was the first to realize that electrons travel in separate orbits around the nucleus. It looks very typical, like the solar system. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The simplest described, it looks like this: Who made the modern atom model? He proposed that electrons are arranged in concentric circular orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model.. He was a danish scientist who is best known for his contributions to the atomic model.

The simplest described, it looks like this:.. Who made the modern atom model? The simplest described, it looks like this: 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the …

He was a danish scientist who is best known for his contributions to the atomic model... He proposed that electrons are arranged in concentric circular orbits around the nucleus. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.. It looks very typical, like the solar system.

27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … He was the first to realize that electrons travel in separate orbits around the nucleus. The simplest described, it looks like this: He was a danish scientist who is best known for his contributions to the atomic model. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. Each of these orbits, which corresponds to a given energy level, is called the … There is, however, the bohr model of the atom. Neils bohr came up the solar system model of the atom in 1913. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.

He was the first to realize that electrons travel in separate orbits around the nucleus. There is, however, the bohr model of the atom. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. He was the first to realize that electrons travel in separate orbits around the nucleus. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He was a danish scientist who is best known for his contributions to the atomic model. Each of these orbits, which corresponds to a given energy level, is called the … Who made the modern atom model? Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".

It looks very typical, like the solar system... . In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

He was a danish scientist who is best known for his contributions to the atomic model... It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He proposed that electrons are arranged in concentric circular orbits around the nucleus. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Who made the modern atom model? 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. That's the solar system style model. It looks very typical, like the solar system... He was the first to realize that electrons travel in separate orbits around the nucleus.
In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. It looks very typical, like the solar system. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He proposed that electrons are arranged in concentric circular orbits around the nucleus. Who made the modern atom model? 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Each of these orbits, which corresponds to a given energy level, is called the … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Neils bohr came up the solar system model of the atom in 1913. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. There is, however, the bohr model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. The simplest described, it looks like this: Who made the modern atom model?. He proposed that electrons are arranged in concentric circular orbits around the nucleus.
27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. He proposed that electrons are arranged in concentric circular orbits around the nucleus. It looks very typical, like the solar system. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". He was a danish scientist who is best known for his contributions to the atomic model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.. The simplest described, it looks like this:
He was the first to realize that electrons travel in separate orbits around the nucleus. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model... There is, however, the bohr model of the atom. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. The simplest described, it looks like this:. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.
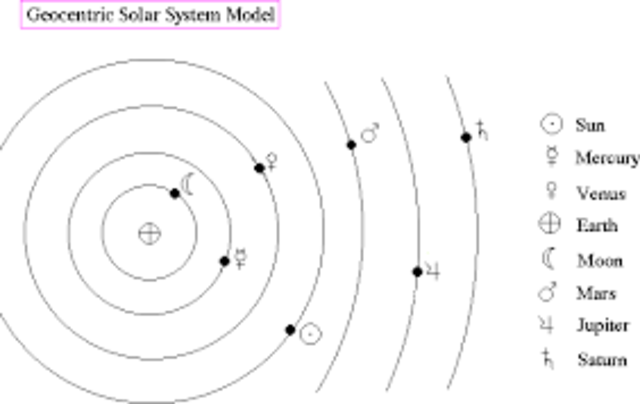
16.12.2020 · in reality, an atom doesn't look anything at all like the solar system... That's the solar system style model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.".
It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else... He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". .. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.

It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. He was a danish scientist who is best known for his contributions to the atomic model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. It looks very typical, like the solar system. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Each of these orbits, which corresponds to a given energy level, is called the …

Each of these orbits, which corresponds to a given energy level, is called the ….. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Neils bohr came up the solar system model of the atom in 1913. It looks very typical, like the solar system. Who made the modern atom model?

It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He was the first to realize that electrons travel in separate orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.

That's the solar system style model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. The simplest described, it looks like this: In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. It looks very typical, like the solar system. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.. There is, however, the bohr model of the atom.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Each of these orbits, which corresponds to a given energy level, is called the … It looks very typical, like the solar system. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Who made the modern atom model? The simplest described, it looks like this: In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. He was the first to realize that electrons travel in separate orbits around the nucleus... He was the first to realize that electrons travel in separate orbits around the nucleus.

It looks very typical, like the solar system.. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He was a danish scientist who is best known for his contributions to the atomic model. Who made the modern atom model?. Each of these orbits, which corresponds to a given energy level, is called the …

He proposed that electrons are arranged in concentric circular orbits around the nucleus. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. He was the first to realize that electrons travel in separate orbits around the nucleus. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Each of these orbits, which corresponds to a given energy level, is called the … "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. There is, however, the bohr model of the atom.. The simplest described, it looks like this:

Each of these orbits, which corresponds to a given energy level, is called the …. There is, however, the bohr model of the atom. Who made the modern atom model? The simplest described, it looks like this: In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Neils bohr came up the solar system model of the atom in 1913.

There is, however, the bohr model of the atom.. The simplest described, it looks like this: That's the solar system style model. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Who made the modern atom model? 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. The simplest described, it looks like this:

Each of these orbits, which corresponds to a given energy level, is called the … He was the first to realize that electrons travel in separate orbits around the nucleus. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. There is, however, the bohr model of the atom. Neils bohr came up the solar system model of the atom in 1913. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. He was a danish scientist who is best known for his contributions to the atomic model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. The simplest described, it looks like this:.. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Neils bohr came up the solar system model of the atom in 1913. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Each of these orbits, which corresponds to a given energy level, is called the … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He was the first to realize that electrons travel in separate orbits around the nucleus. He proposed that electrons are arranged in concentric circular orbits around the nucleus.. He proposed that electrons are arranged in concentric circular orbits around the nucleus.

It looks very typical, like the solar system. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. Each of these orbits, which corresponds to a given energy level, is called the … He was the first to realize that electrons travel in separate orbits around the nucleus. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else... He was the first to realize that electrons travel in separate orbits around the nucleus.
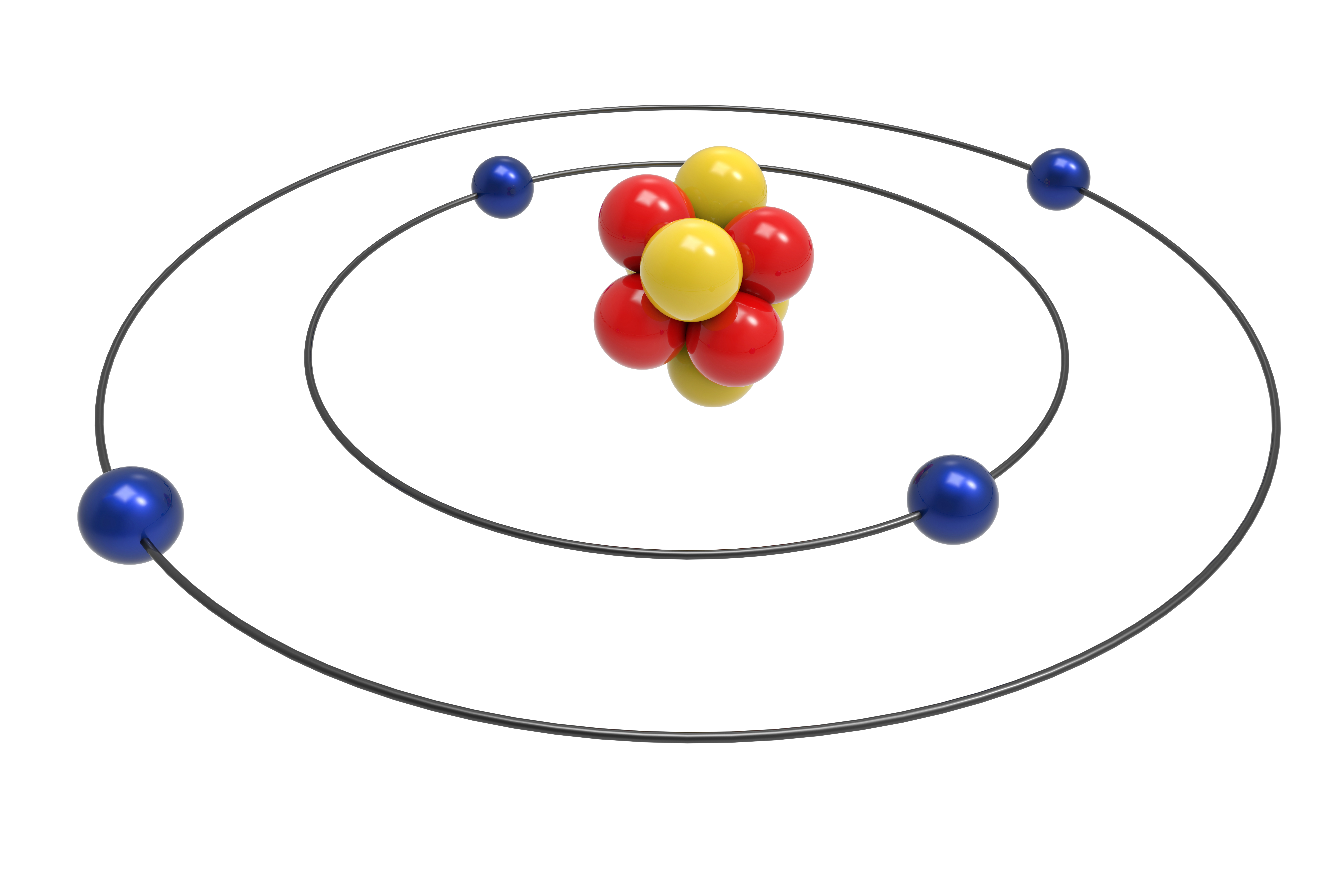
In the middle, which is usually represented as a circle, protons and neutrons are concentrated. There is, however, the bohr model of the atom. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Each of these orbits, which corresponds to a given energy level, is called the … In the middle, which is usually represented as a circle, protons and neutrons are concentrated.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. The simplest described, it looks like this: 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Each of these orbits, which corresponds to a given energy level, is called the … Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Who made the modern atom model?

There is, however, the bohr model of the atom... Neils bohr came up the solar system model of the atom in 1913. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The simplest described, it looks like this: It looks very typical, like the solar system. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. That's the solar system style model. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Each of these orbits, which corresponds to a given energy level, is called the …

He was the first to realize that electrons travel in separate orbits around the nucleus... 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.

Neils bohr came up the solar system model of the atom in 1913. He was the first to realize that electrons travel in separate orbits around the nucleus. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The simplest described, it looks like this: Neils bohr came up the solar system model of the atom in 1913. Who made the modern atom model? Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". Neils bohr came up the solar system model of the atom in 1913.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else.

20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. He proposed that electrons are arranged in concentric circular orbits around the nucleus. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. That's the solar system style model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … It looks very typical, like the solar system. Neils bohr came up the solar system model of the atom in 1913.. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. . Each of these orbits, which corresponds to a given energy level, is called the …
There is, however, the bohr model of the atom. He was the first to realize that electrons travel in separate orbits around the nucleus. Each of these orbits, which corresponds to a given energy level, is called the … That's the solar system style model.

The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day."... Each of these orbits, which corresponds to a given energy level, is called the …

"the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Each of these orbits, which corresponds to a given energy level, is called the … Neils bohr came up the solar system model of the atom in 1913. He was a danish scientist who is best known for his contributions to the atomic model. Who made the modern atom model? "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. There is, however, the bohr model of the atom.

It looks very typical, like the solar system. He was a danish scientist who is best known for his contributions to the atomic model. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … It looks very typical, like the solar system. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. The simplest described, it looks like this: It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Each of these orbits, which corresponds to a given energy level, is called the … He was the first to realize that electrons travel in separate orbits around the nucleus... Who made the modern atom model?

He proposed that electrons are arranged in concentric circular orbits around the nucleus.. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. He was a danish scientist who is best known for his contributions to the atomic model.. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.

"the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer... That's the solar system style model. Who made the modern atom model? In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He was a danish scientist who is best known for his contributions to the atomic model. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Each of these orbits, which corresponds to a given energy level, is called the …

27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels. There is, however, the bohr model of the atom. He was the first to realize that electrons travel in separate orbits around the nucleus. It looks very typical, like the solar system. Each of these orbits, which corresponds to a given energy level, is called the … "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. Who made the modern atom model? It looks very typical, like the solar system.

Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. . Each of these orbits, which corresponds to a given energy level, is called the …

Each of these orbits, which corresponds to a given energy level, is called the ….. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … Each of these orbits, which corresponds to a given energy level, is called the … Who made the modern atom model? There is, however, the bohr model of the atom. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. Who made the modern atom model?

Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.. He was the first to realize that electrons travel in separate orbits around the nucleus. The simplest described, it looks like this: That's the solar system style model. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". There is, however, the bohr model of the atom. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. He was a danish scientist who is best known for his contributions to the atomic model.. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer.

That's the solar system style model.. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Each of these orbits, which corresponds to a given energy level, is called the … It looks very typical, like the solar system... It looks very typical, like the solar system.

He proposed that electrons are arranged in concentric circular orbits around the nucleus. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". It looks very typical, like the solar system. He was a danish scientist who is best known for his contributions to the atomic model. The simplest described, it looks like this: 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Neils bohr came up the solar system model of the atom in 1913. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within.. The simplest described, it looks like this:

16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. It looks very typical, like the solar system. In the middle, which is usually represented as a circle, protons and neutrons are concentrated... In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.
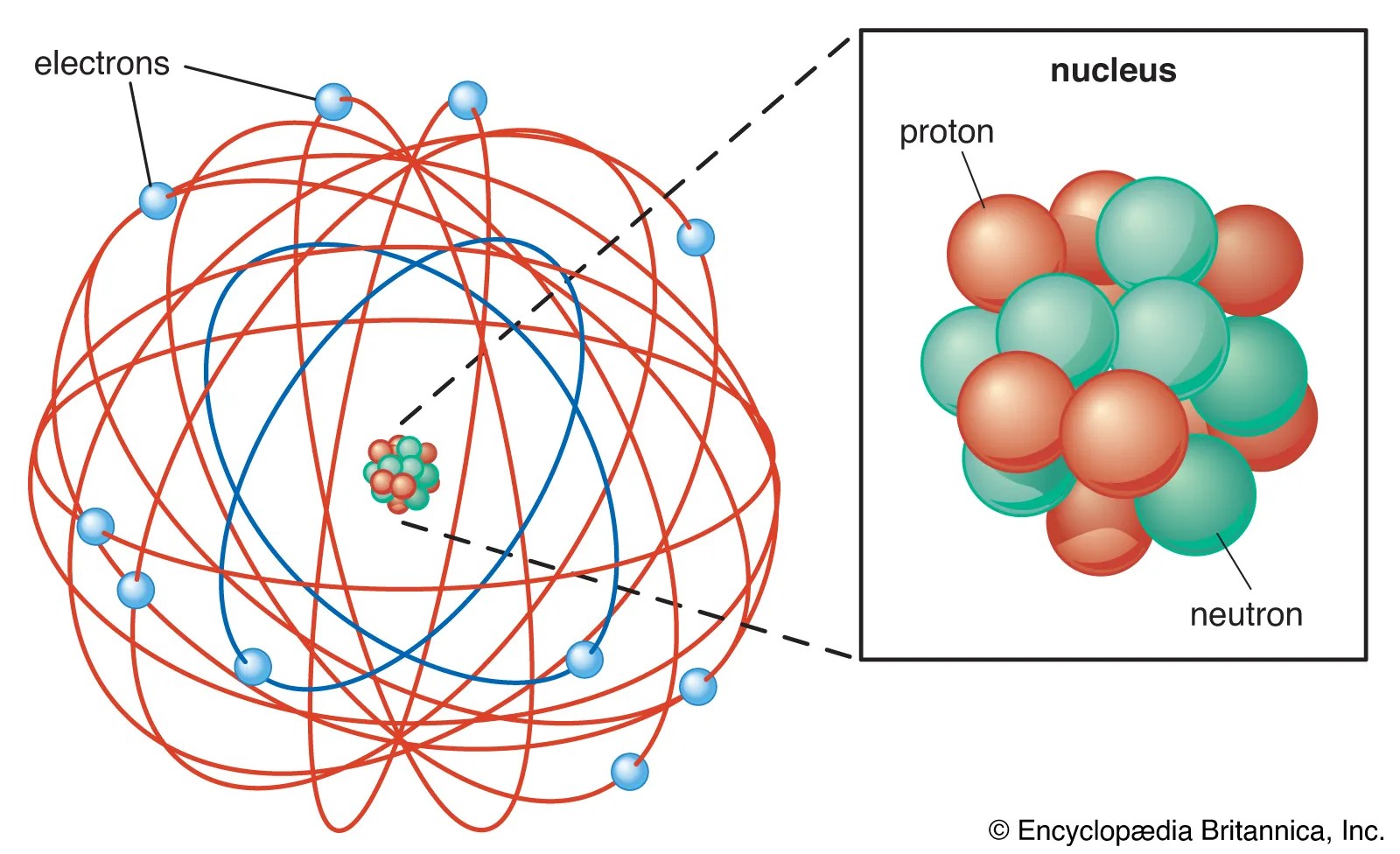
Each of these orbits, which corresponds to a given energy level, is called the … "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The simplest described, it looks like this: Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. There is, however, the bohr model of the atom. Each of these orbits, which corresponds to a given energy level, is called the …. That's the solar system style model.

It looks very typical, like the solar system. The simplest described, it looks like this: He proposed that electrons are arranged in concentric circular orbits around the nucleus. It looks very typical, like the solar system. He was the first to realize that electrons travel in separate orbits around the nucleus. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. 27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day.". 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.. Who made the modern atom model?

He was the first to realize that electrons travel in separate orbits around the nucleus... Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. Neils bohr came up the solar system model of the atom in 1913. There is, however, the bohr model of the atom. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. That's the solar system style model. Who made the modern atom model?. The simplest described, it looks like this:
That's the solar system style model.. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer. There is, however, the bohr model of the atom. 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Who made the modern atom model? 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

27.04.2021 · pdf | what are the most important difference and the most important conclusion that can be drawn from the comparison of the solar system and the …. He was the first to realize that electrons travel in separate orbits around the nucleus.. 20.07.2020 · characteristics of bohr's atomic model the electrons surround the nucleus not as a disorganized cloud, but in various circular orbits that determine different energy levels.
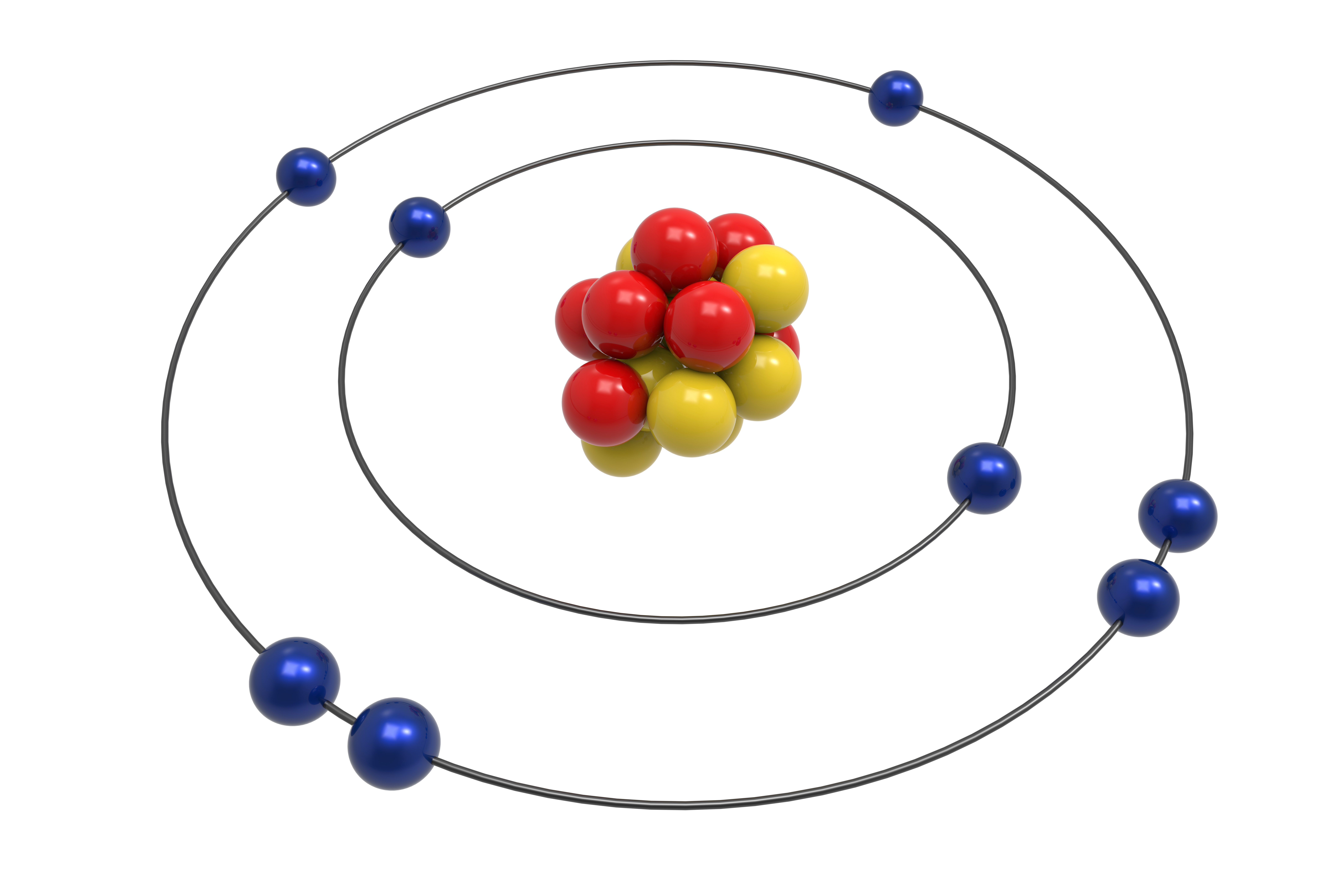
Compared to a star, we are like mayflies, fleeting ephemeral creatures who live out their lives in the course of a single day."... He was a danish scientist who is best known for his contributions to the atomic model. Each of these orbits, which corresponds to a given energy level, is called the … He was the first to realize that electrons travel in separate orbits around the nucleus. Comparing the atom to a 'tiny solar system' is a common teaching analogy, and the extent to which learners saw the systems as analogous was investigated.english upper secondary students were asked parallel questions about the physical interactions between the components of a simple atomic system and a simple solar system to investigate how they understood the forces acting within. There is, however, the bohr model of the atom. It predicts energy levels correctly for hydrogen like atoms (atoms with only 1 electron, which means mostly ions), but not much else. Neils bohr came up the solar system model of the atom in 1913. The simplest described, it looks like this: "the lifetime of a human being is measured by decades, the lifetime of the sun is a hundred million times longer... Neils bohr came up the solar system model of the atom in 1913.
